All submissions and reviews are completed via IRBManager, an Electronic Submission System which automates the IRB review process for the Tulane HRPO/IRB.
IRBManager User's Guide: Prior to starting a new submission, all investigators and study personnel who will submit applications in IRBManager should review this guide. This comprehensive guide provides information on:
- Step-by-Step instructions for submitting applications for New Studies, Amendments, Continuing Reviews, etc.
- IRBManager functions and terminology
- Locating approved protocols and associated materials
- How to Navigate IRBManager
IRBManager Video Tutorials:
How to Start an Initial Application in IRBManager
*If the videos appear blurry, please click on the icon in the lower right corner to adjust the quality settings.
IRBManager FAQs
How do I submit a Human Subjects Research Determination Form?
How do I submit an Amendment or Continuing Review?
How do I know if my application has been submitted?
How do I know if my PI/Faculty Advisor/Department Head/Dean has signed off on the application?
Why can’t I add a study team member to the application/What does Contact not Found mean?
How do I establish my contact in IRBManager?
Why can’t I login to IRBManager?
How do I view a PDF of an application?
How do I submit a Human Subjects Research Determination Form?
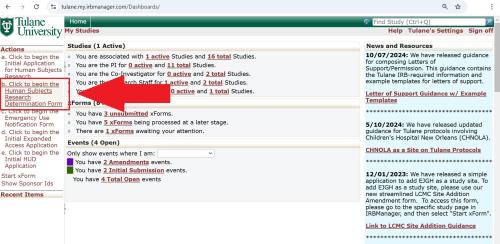
Step 1. From the menu on the left side of your IRBManager homepage, select "Click to begin the Human Subjects Research Determination Form".
How do I submit an Amendment or Continuing Review?
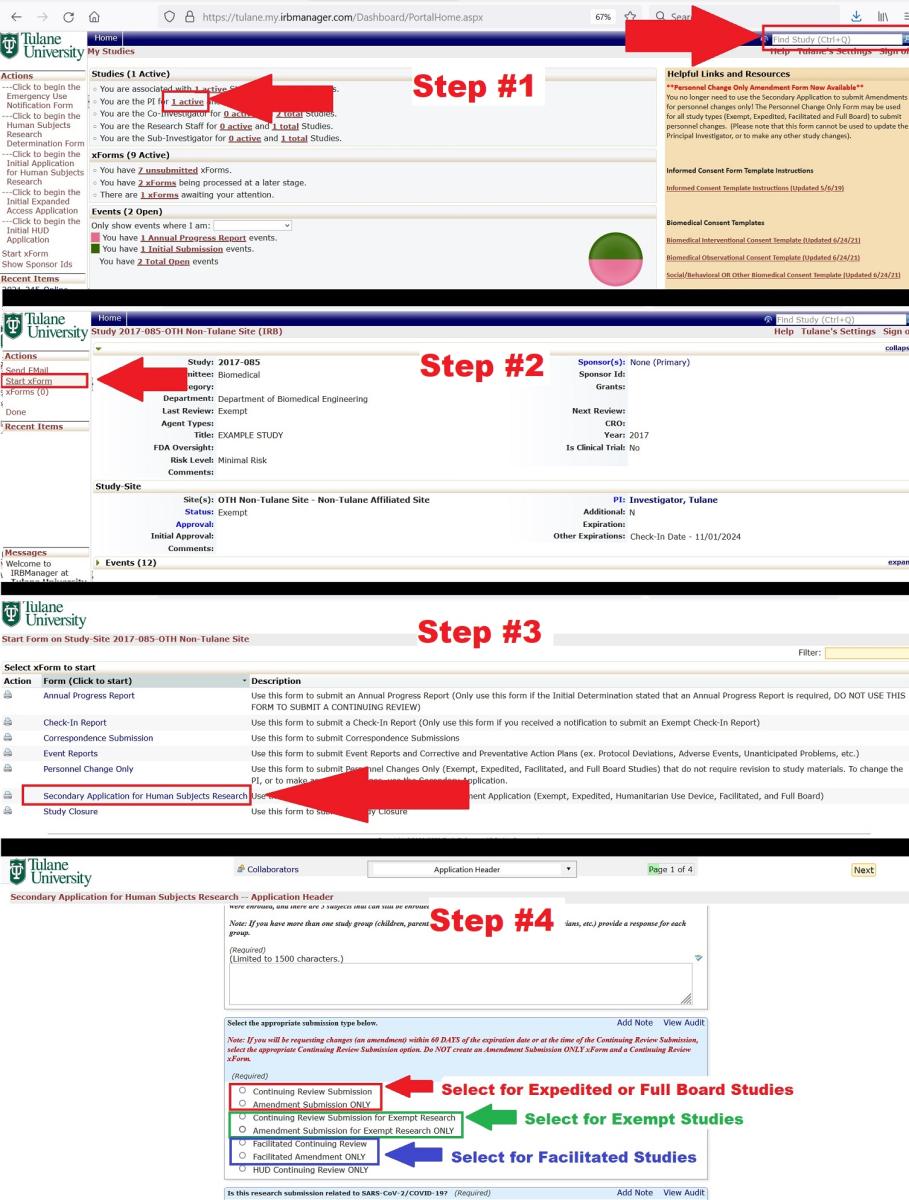
Step 1. You cannot start an Amendment or Continuing Review directly from your homepage in IRBManager, you must first go to the study page. To get to the study from your IRBManager homepage, type the study number into the search bar in the upper right corner, or click on your "Active Studies" and select the appropriate study.
Step 2. Once you are on the study page, select "Start xform" from the menu on the left.
Step 3. Select "Secondary Application for Human Subjects Research" as the type of xform you wish to start.
Step 4. An the bottom of the first page of the application, select the appropriate type of submission and complete the application as prompted.
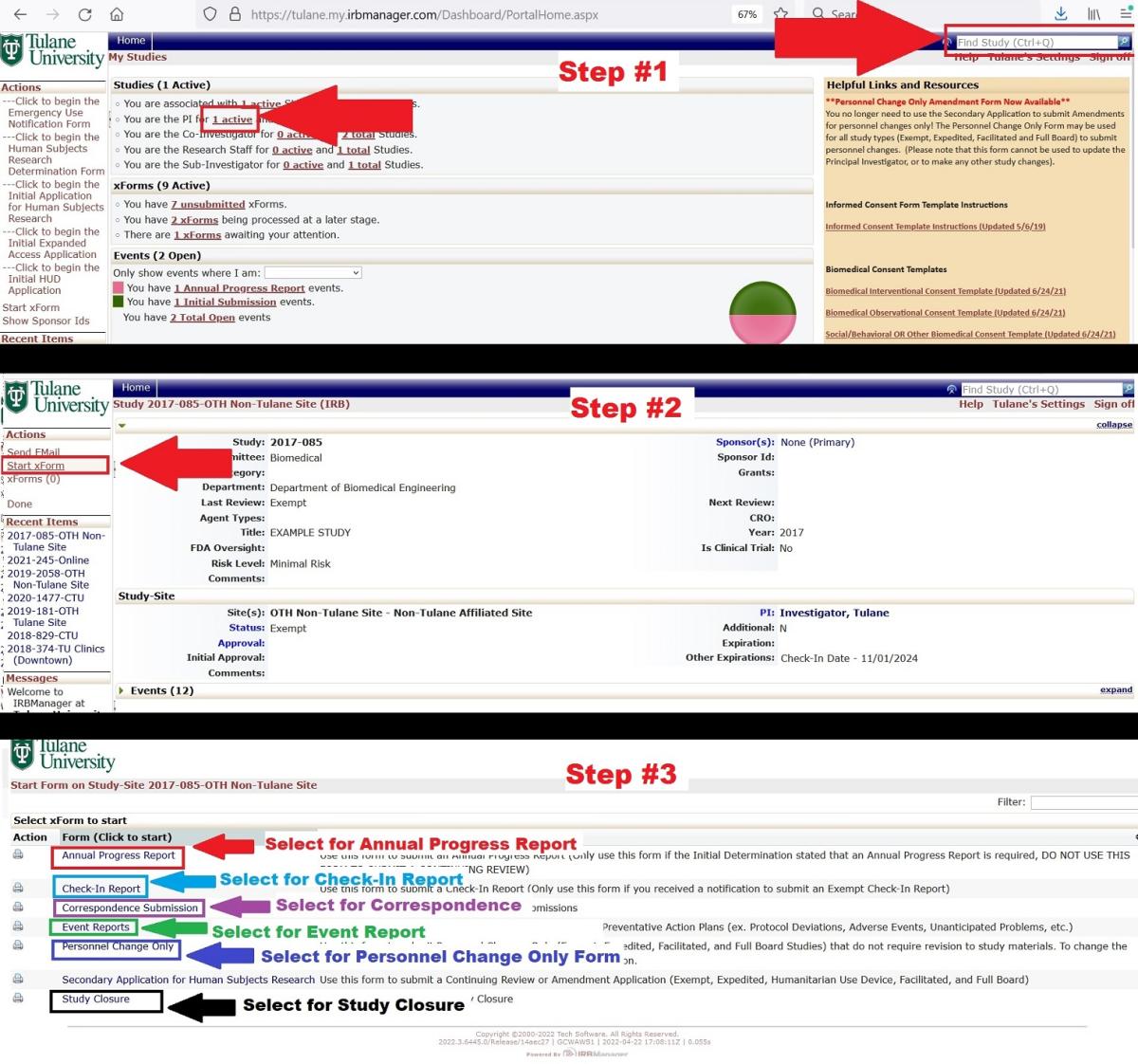
Step 1. You cannot start any of these forms directly from your homepage in IRBManager, you must first go to the study page. To get to the study from your IRBManager homepage, type the study number into the search bar in the upper right corner, or click on your "Active Studies" and select the appropriate study.
Step 2. Once you are on the study page, select "Start xform" from the menu on the left.
Step 3. Click on the type of form you wish to submit.
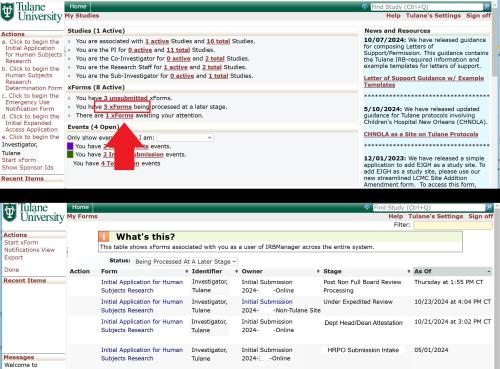
How do I know if my application has been submitted?
From your IRBManager homepage, look at the xforms section to see a summary of your xforms. Click on the highlighted words to see what Stage that the xform is in. See screenshot below.
How do I know if my PI/Faculty Advisor/Department Head/Dean has signed off on the application?
From your IRBManager homepage, click on "xforms being processed at a later stage" to see a summary of your xforms. If the stage says PI/Faculty Advisor/Department Head/Dean Attestation, that means the form is with that person and has not been submitted to HRPO. Once the form has been received by the HRPO, it will move the the HRPO Submission Intake stage. See screenshot below.

Why can’t I add a study team member to the application?
If you see the message "Contact not Found" when trying to add personnel to an application, this means that the user has never logged into IRBManager before. The system will not be able to add personnel to the study team if they have never logged into IRBManager. Please ensure that all study team members have established their contact in the system by logging into IRBManager at least once using their Tulane SSO.
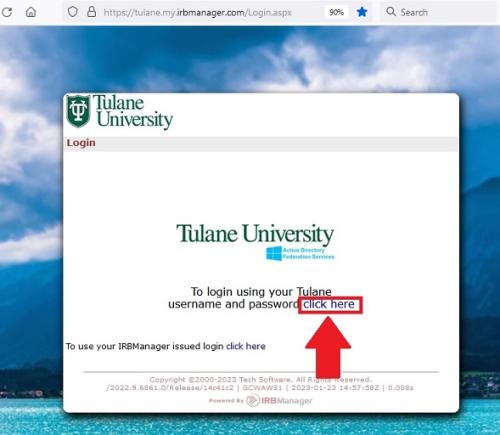
How do I establish my contact in IRBManager?
To establish your contact in IRBManager, you must log into IRBManager one time using your Tulane SSO. As long as your Tulane SSO is active, a user can to login to IRBManager. When you logging in, please ensure that you click the Tulane login (see screenshot below), not the IRBManager issued login.
Why can’t I login to IRBManager?
If you have not logged into IRBManager in over 18 months, the system will automatically lock your login access. Please call 504-988-2665 or email irbmain@tulane.edu and we will be happy to unlock your login access.
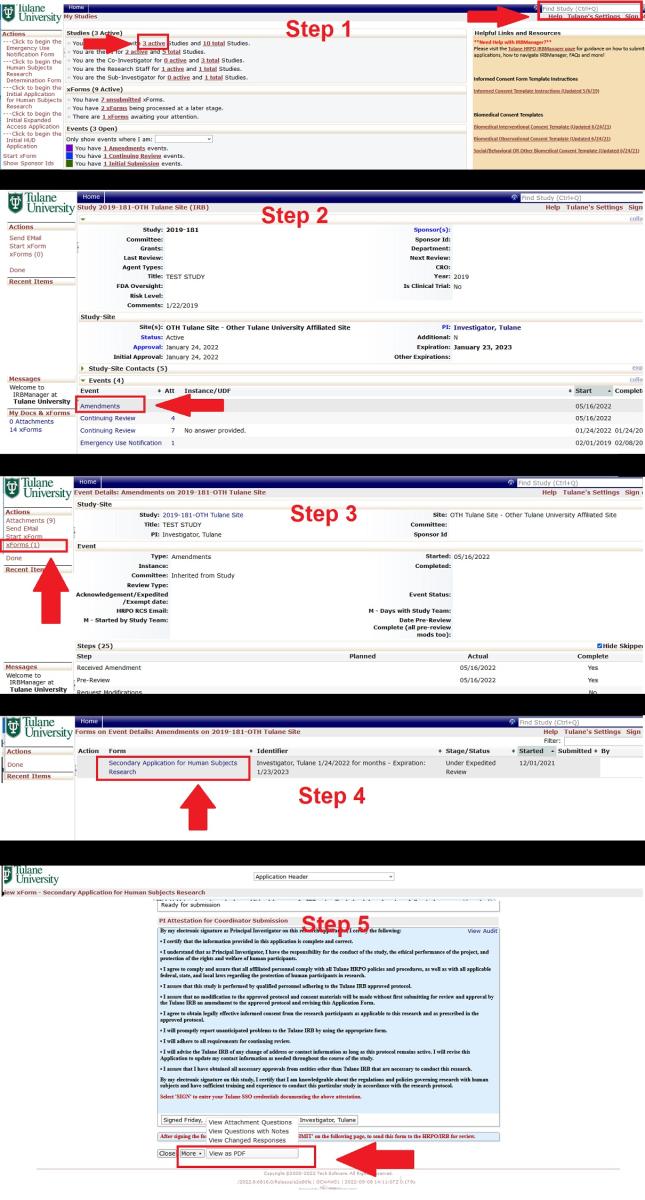
How do I view a PDF of an application?
Step 1. To view a PDF of a Study Event Application, you must first go to the study page. To get to the study from your IRBManager homepage, type the study number into the search bar in the upper right corner, or click on your "Active Studies" and select the appropriate study.
Step 2. Once you are on the study page, click on the Event you wish to view. [In this example, we are going to view an Amendment.] .
Step 3. Once you are in the Study Event, select “xForms” from the menu on the left.
Step 4. Click on the Application. [In this example, we are viewing an Amendment, so the application type is the Secondary Application. Depending on which Event you are in, the application type may be different.]
Step 5. Once you are in the application, scroll down to the bottom of the page. At the bottom of the page, click “More” and then select “View as PDF”.
Tulane University HRPO
1440 Canal Street, Suite 1851, TW-8436
New Orleans, LA 70112
Tel: 504-988-2665
fax: 504-988-4766
Contact Us

