Before beginning any Human Subjects research, all research personnel must complete the appropriate Tulane University CITI Training Course(s). The Tulane IRB does not accept CITI courses from other institutions. The CITI courses can be completed at: www.citiprogram.org
After registering on the CITI website, add Tulane University as your Affiliation and select the applicable courses (listed below).
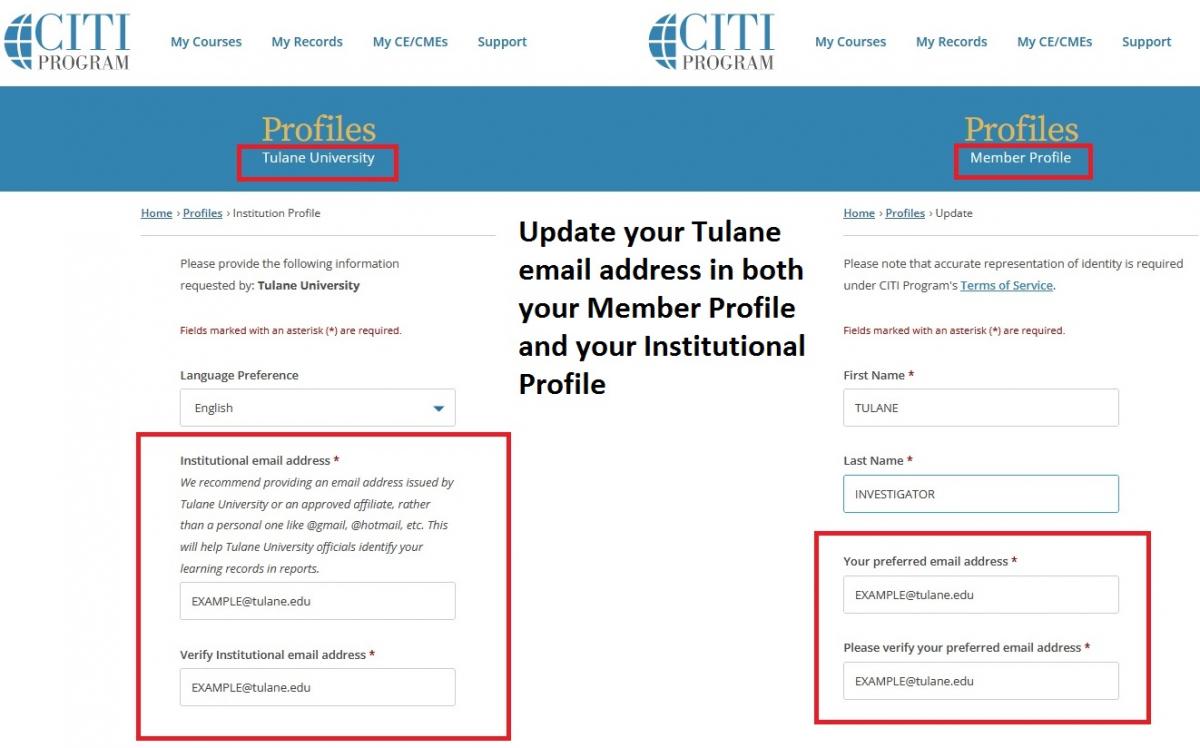
Please ensure that you enter your Tulane email address in TWO places in your CITI account. If you do not use your Tulane email address in both sections, your CITI training information will not automatically populate in IRBManager. In your Member Profile: Enter your Tulane email address as your “Preferred Email”. In your Institutional Profile: Enter your Tulane email address as your “Institutional email address”.
For Biomedical Studies, all study team members (including the Faculty Advisor if the PI is a student) must complete ONE of the following Tulane CITI courses:
- Group 1 Biomedical Research Investigators and Key Personnel Course
- Good Clinical Practice (GCP) Course
- GCP for Clinical Trials with Investigational Drugs and Biologics (ICH Focus)(NIH requirements) Course
- GCP for Clinical Trials with Investigational Drugs and Medical Devices (U.S. FDA Focus)
For Social Behavioral Studies, all study team members (including the Faculty Advisor if the PI is a student) should complete the Tulane Group 2 Social and Behavioral Research Investigators and Key Personnel CITI Course. Please note that for Social Behavioral studies, the IRB can accept any of the Biomedical Courses listed above in place of the Group 2 Social and Behavioral Research Investigators and Key Personnel course.
For both Biomedical and Social Behavioral Studies, all investigators (PI, Co-I, Sub-I) must also complete the Conflict of Interest (COI) CITI Course.
