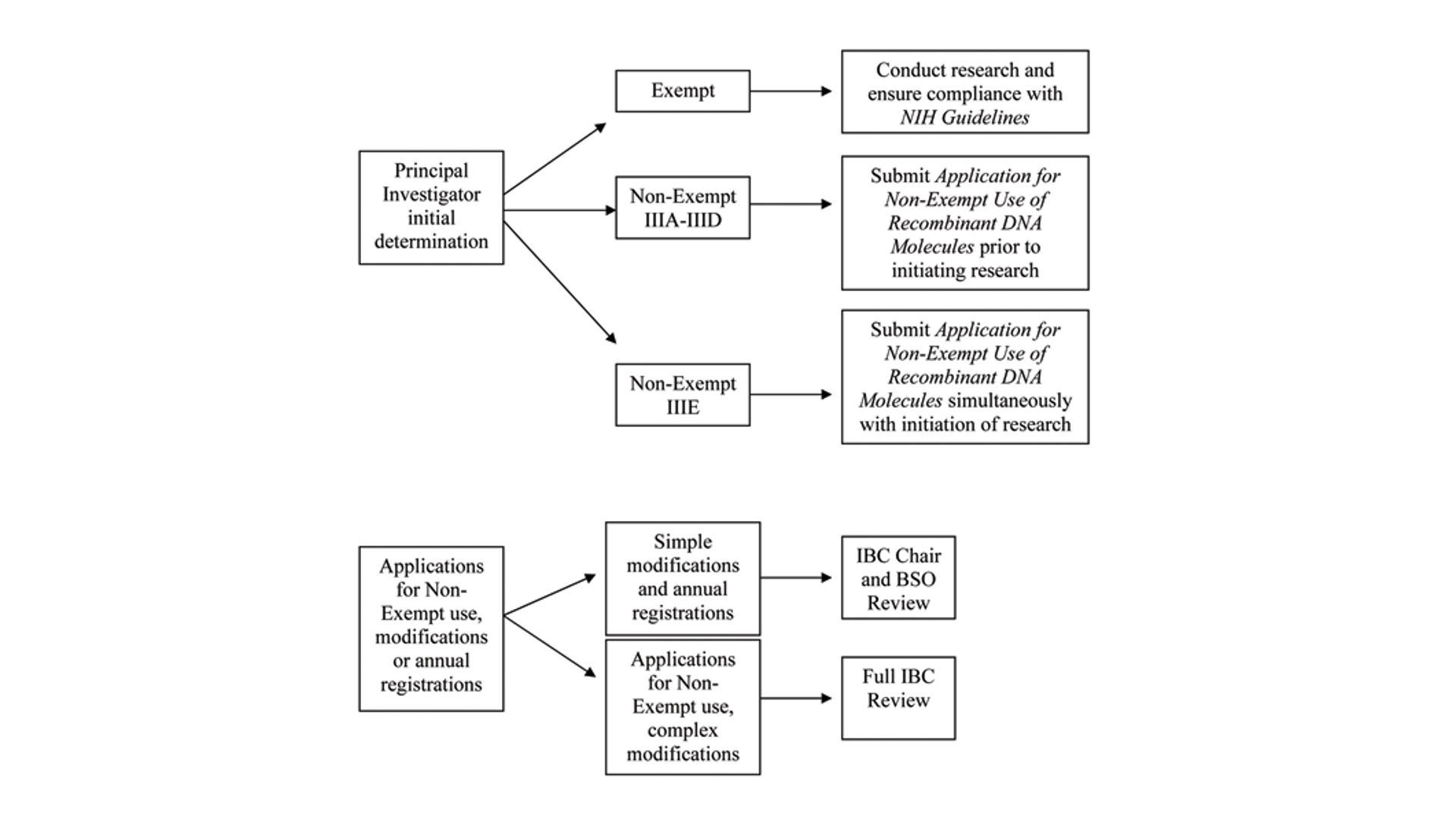
The Principal Investigator must make an initial determination whether the subject research is Exempt or Non-Exempt under the NIH Guidelines. When a completed IBC Protocol Form is received, it is date-stamped and assigned a new or existing protocol identification number that will be referenced on all subsequent IBC correspondence. This number is provided to the Principal Investigator in an electronic memo confirming IBC receipt of the protocol. The full IBC will review all submitted applications. The protocol form provides links for information about the types of research activities that require full committee review.
Additional steps are necessary for studies involving whole animals or clinical trials. For whole animal studies in which the animal's genome is to be altered by stable introduction of recombinant DNA, or DNA derived therefrom, into the germ-line (transgenic animals) and experiments involving introduction of recombinant DNA or viable recombinant DNA-modified microorganisms tested on whole animals, simultaneous IBC and IACUC approval must be obtained (or an IACUC approval number should be provided). There are no Exempt protocols in this category and all such procedures require IBC approval before initiation. The purchase or transfer of most transgenic rodents is exempt under NIH Guidelines and need not be registered with the IBC unless these animals are being exposed to rDNA constructs or viable recombinant DNA-modified microorganisms. All IBC applications that involve whole animals must include detailed descriptions of constructs to be administered to the animals, appropriate biocontainment, and any potential risks to researchers, animal handlers, or other research animals.
Experiments involving the deliberate transfer of recombinant or synthetic nucleic acid molecules or DNA, or RNA derived from recombinant or synthetic DNA, into one or more human research participants require IBC and IRB Approvals and RAC Review before research participant enrollment. In addition to the competed IBC protocol form, Principal Investigators must submit a) the Clinical Protocol, b) the Investigator's Brochure and c) informed consent forms to the IBC. Gene Transfer study applications must also be accompanied by a completed Appendix M, Points to Consider in the Design and Submission of Protocols for the Transfer of Recombinant DNA Molecules into the Genome of One or More Human Subjects from the NIH Guidelines. The IBC must assure that all aspects of Appendix M have been appropriately addressed by the Principal Investigator before submission to the RAC. Final IBC approval is granted only after the RAC review process has been completed (see Appendix M-I-B, RAC Review Requirements).
